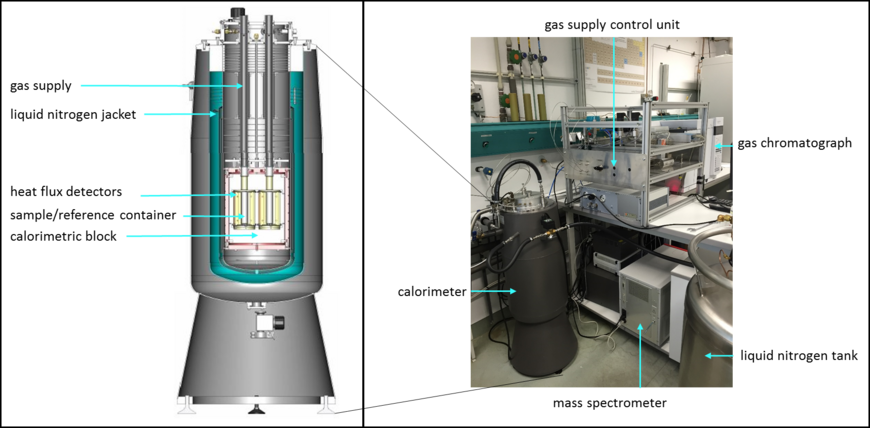
Equipment & location: B362
Measurement Principle
Differential scanning calorimetry (DSC) is a method of thermal analysis for determining the amount of heat released or consumed by a sample at a defined temperature change. For this purpose a container with sample material and an empty reference container are simultaneously exposed to the same temperature regime. During the investigated process (phase changes and/or chemical reactions) energy is consumed or released by the sample. These exothermic or endothermic processes are mirrored by temperature differences between the containers of sample and reference.
With the DSC, this temperature difference is used to conclude on enthalpy changes (heat flow) by integrating the ΔT-TRef curve.
Seteram BT 2.15 calorimeter
The BT 2.15 calorimeter includes a controlled heatable calorimetric block which holds sample and reference container. The containers of 14 ml volume, are surrounded by heat flow detectors. For cooling, the device is equipped with a liquid nitrogen supply. Temperature adjustment and measurement occurs via electrical and pneumatic control units.
With the BT 2.15 calorimeter, processes can be investigated in a temperature range of -196°C to 200°C with sensitive cooling and heating rates.
The measurement range down to very low temperatures, enables the thermometric study of gas hydrate related processes.
Depending on the sample container used, investigations can be carried out at atmospheric pressure but also at elevated pressure and the use of specific gases. The additional installation of a gas regulator unit allows for accurate pressure settings and gas flow rates. The gas composition can be quasi-continuously monitored during an experiment using the coupled downstream gas analysis (GC, MS). By this means, gas-induced reactions can be chemically and thermally evaluated.