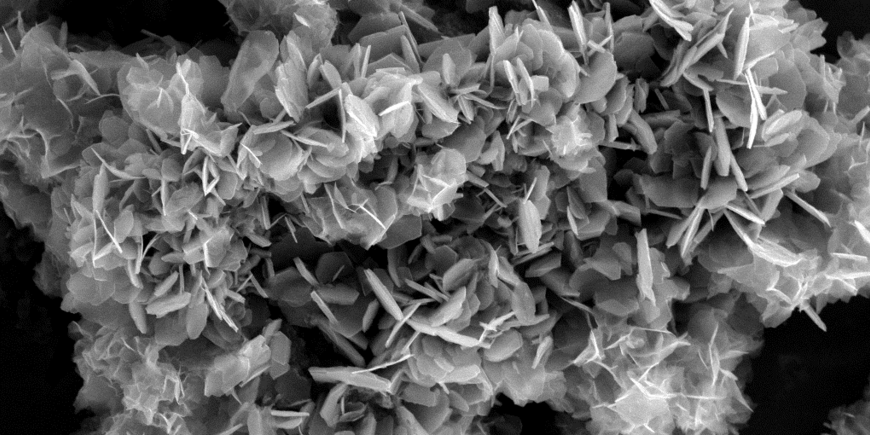
Reactions at mineral surfaces greatly influence several key (bio)geochemical processes including biomineralization, nutrient and trace element cycling and contaminant dynamics. In this research theme, we focus on the mechanisms and kinetics of mineral nucleation and growth, and how these processes impact the speciation, sequestration or release/transport of various elements in Earth’s surface and (near-)subsurface environments. Currently, we are interested in the formation and/or transformation of calcium carbonates and sulfates, clay minerals, iron (oxyhydr)oxides and sulfides and phosphates (e.g., struvite) in natural and engineered environments.
In order to understand the key controlling factors affecting mineral formation and/or transformation, we make synthetic analogues of these mineral phases and perform experiments under simulated natural conditions. Such an approach allows us to perform in-depth investigations on the how minerals form and break, and how these impact critical (bio)geochemical cycles (e.g., iron, phosphorous, sulfur) as well as the mobility and toxicity of metals and metalloids (e.g., arsenic, chromium, nickel).
We integrate several analytical approaches from inorganic chemistry, materials chemistry and nanoscience to help us gain better understanding on their mineral chemistry. Specifically, we use a suite of laboratory-based solid state and aqueous phase characterization techniques (e.g., TEM, SEM, XRD, IR, ICP-OES/MS, IC) and combine them with synchrotron-based scattering (e.g., SAXS/WAXS, PDF), and spectroscopic (e.g., SXM, XAFS) techniques to probe these reactions at high spatial or temporal resolution.
Current research projects under this research theme include:
- Clay Mineral Formation at Ambient Conditions
- High-Energy X-ray Scattering Studies on Mineral Crystallization
- Experimental Diagenesis and Mineral Transformation Phenomena of Sedimentary Carbonates and Sulphates
- Iron Minerals and their Impact on Nutrients and Trace Elements Cycling
- Struvite Crystallization
Our research is part of a large comparative sampling effort, in cooperation with other universities and institutions that aims to unravel the complex interplay between light absorbing impurities (LAI; e.g., microbes, mineral dust, black/brown carbon, etc.), and glacial ecosystems. In particular, we are interested in how LAI affect glacial melt rates (their impact on albedo), and their fate following melt events. We then hope to use these data to contribute to larger global melt models that attempt to predict climate change in the future.
The biodiversity of these glacial ecosystems will be explored using a variety of ‘omic’ techniques. These include targeted amplicon sequencing of ribosomal genes, metagenomic and metagenomic sequencing to infer microbial physiology, and mass spectrometry of metabolites in order to determine downstream metabolic processes. We are particularly interested in the role that snow and ice algae play in glacial ecosystem processes. Previous research has shown that algae can reduce albedo by up to 13%, which can dramatically increase melting. We’ve also documented that when melting starts, snow and later ice algae are the main primary photosynthetic organisms that bloom on glacier surfaces.
Additionally, we are interested in the geochemistry of these environments as it is very important in determining the types of organisms that are able to survive and thrive. Assessing the geochemistry can provide a picture of nutrients available on glacial surfaces which may be bioavailable for growth processes. Analysis of the geochemistry of the system is accomplished by evaluating cations and anions in snow, ice, and meltwaters.
Furthermore, algal blooms can produce large amounts of organic matter, however we understand little about the processes that control the production of organic compounds nor how and if they are further degraded by heterotrophic organisms or become exported through rivers to fuel ocean productivity. Assessing the carbon inputs to the system (anthropogenic or otherwise) can also help us to determine the carbon sources available to drive biological processes. Analyses are performed on glacial samples in order to determine dissolved and particulate organic matter composition. This data provides us with a comprehensive view of all organic and inorganic inputs through air and by snow to the surface of these glacial ecosystems.
Such combined microbial diversity and organic carbon data are crucial if we want to understand and quantify the fundamental processes leading to glacier melting and derive data sets that will be implemented in global numerical climate models.
Current research projects under this research theme include:
Electron microscopy allows characterization of geomaterials at the microscopic to nano-scale. We specialize in the characterization of a wide-range of minerals including, but not limited to, silicates, (oxyhydr)oxides, sulfides, sulfates, carbonates and phosphates. In addition, we are developing novel high-resolution electron imaging and spectroscopic tools, as well as complex sample environments, for the characterization of geomaterials.
The research group runs and manage the Potsdam Imaging and Spectral Analysis (PISA) Facility. The PISA facility currently houses 2 high-resolution Transmission Electron Microscopes (TEM), 1 high-resolution Scanning Electron Microscope (SEM) and 2 Focused Ion Beam combined with SEM (FIB-SEM).
Using our ZEISS Ultra Plus and FEI Quanta SEMs and associated Energy Dispersive X-ray (EDX) Spectroscopy, we can provide mineral surface structure and its chemical composition. Further mineral characterization of thin samples can be obtained using Transmission Electron Microscopy (TEM) and Scanning Transmission Electron Microscopy (STEM) on our FEI Tecnai and Thermo Fisher Scientific Titan Themis Z TEMs. On these TEMs, Energy Dispersive X-ray (EDX) Spectroscopy and Selected Area Electron Diffraction (SAED) can be used to provide chemical composition and crystal structure at the nano-scale. Focused Ion Beam (FIB) milling on FEI Helios electron microscope allows thin sample preparation for TEM observations. Finally, Liquid Phase Transmission Electron Microscopy (LPTEM) using Poseidon holder (Protochips) is a cutting-edge technique currently developed here to design experimental procedure that will allow us to investigate in situ mineral formation and mineral dissolution processes.
Current research projects from our group include:
- Clay Mineral Formation at Ambient Conditions
- Iron Minerals and their Impact on Nutrients and Trace Elements Cycling
- Development of Liquid Phase Transmission Electron Microscopy for Geosciences
External scientific users can request access to these microscopes. Please visit Potsdam Imaging and Spectral Analysis (PISA) Facility for more information.