Diamonds are fascinating - not only as jewellery having brilliant colours, but also because of the extreme hardness of the material. How exactly this highly valued variant of carbon is formed deep underground and under extremely high pressures and temperatures remains a mystery. Now, researchers from the Russian Academy of Sciences Novosibirsk, collaborating with the German Research Centre for Geosciences Potsdam, have documented a new influencing factor using both theory and experiment. Weak electric fields can be a decisive catalyst for the formation of diamond. They published their findings today in the journal Science Advances.
Diamond, like graphite, is a special form of carbon. Its cubic crystal structure and its strong chemical bonds give it its unique hardness. For thousands of years, it has also been sought after as both a tool and as a thing of beauty. Only in the 1950s did it become possible to produce diamonds artificially for the first time.
Most natural diamonds form in the Earth's mantle at depths of at least 150 kilometres, where temperatures in excess of 1500 degrees Celsius and enormously high pressures of several gigapascals prevail – more than 10.000 times that of a well-inflated bicycle tyre. There are different theories for the exact mechanisms that are responsible for their formation. The starting material is carbonate-rich melts, i.e. compounds of magnesium, calcium or silicon which are rich in both oxygen and carbon.
A new pathway for the formation of diamonds
Because electro-chemical processes take place in the Earth's mantle and the melts and liquids that exist there can have a high electrical conductivity, researchers led by Yuri Palyanov of the V. S. Sobolev Institute of Geology and Mineralogy SB of the Russian Academy of Sciences Novosibirsk developed a model for the formation of diamonds in which highly localised electrical fields play a central role. According to this concept, applying less than even one volt – a voltage lower than that provided by most household batteries - provides electrons that trigger a chemical transformation process. These available electrons make it possible for certain carbon-oxygen compounds of the carbonates to become CO2 through a series of chemical reactions, ultimately leading to pure carbon in the form of diamond.
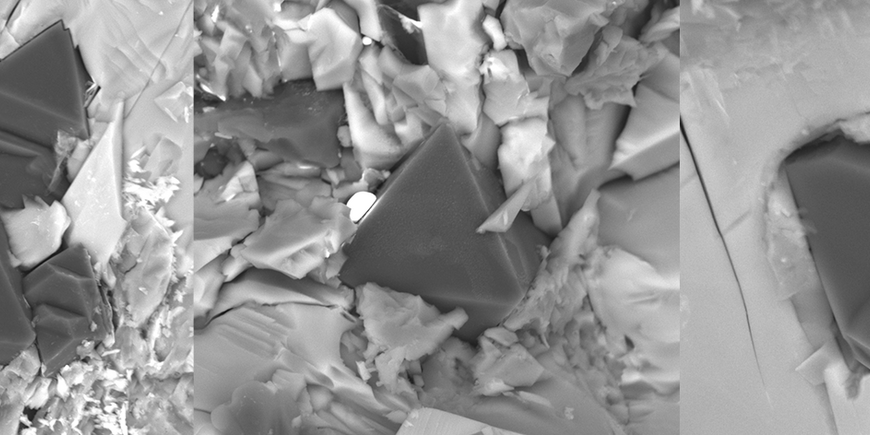
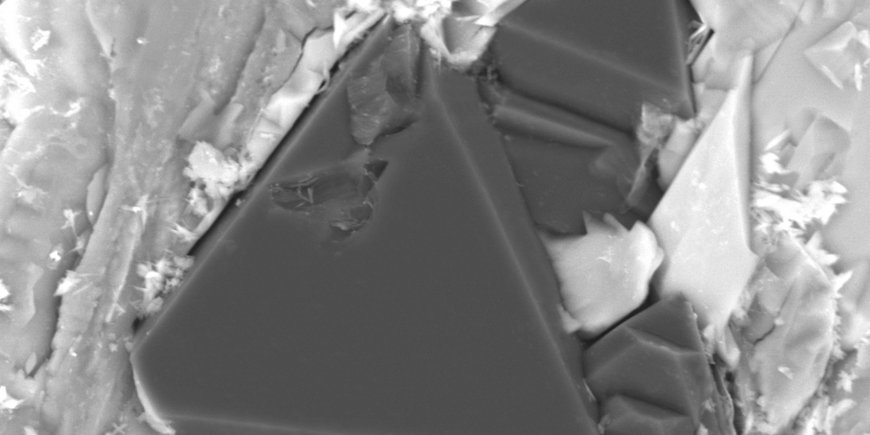
To test their theory, the Russian research team developed a sophisticated experimental facility: A millimetre-sized platinum capsule was surrounded by a heating system which in turn was placed in a high-pressure apparatus needed to produce immense pressures of up to 7.5 gigapascals. Tiny, carefully constructed electrodes led into the capsule, which had been filled with carbonate or carbonate-silicate powders. Numerous experiments were run at temperatures between 1300 and 1600°C, some of which lasted for as long as 40 hours.
Diamonds only grow with voltage
The experiments conducted in Novosibirsk showed, as predicted, that tiny diamonds grow in the vicinity of the negative electrode over the course of several hours, but this happened only when a small voltage was applied; half a volt was already enough. With a diameter reaching a maximum of 200 micrometres, i.e. one fifth of a millimetre, the newly created crystals were smaller than a typical grain of sand. Furthermore, as expected, the other pure-carbon mineral graphite was found to form in experiments conducted at lower pressures. Further proof of the new mechanism came when the researcher reversed the voltage polarity - diamonds then grew on the other electrode, exactly as expected. Without any voltage being suppled from outside the capsule neither graphite nor diamonds formed. In the vicinity of the diamonds, other minerals that are associated with the Earth's deep mantle were also found.
"The experimental facilities in Novosibirsk are absolutely impressive," says Michael Wiedenbeck, head of the SIMS laboratory at the GFZ, which is part of Potsdam’s Modular Earth Science Infrastructure (MESI). He has been cooperating with the Russian researchers for more than ten years; he along with SIMS laboratory engineer Frédéric Couffignal, analysed diamonds produced by their Russian colleagues. In order to determine whether Yuri Palyanov's theory on diamond formation is completely correct, the isotopic composition of the diamonds had to be characterised very precisely.
Precision analysis “made in Potsdam”
The Potsdam researchers used secondary ion mass spectrometry (SIMS) for this purpose. The Potsdam instrument is a highly specialized mass spectrometer, providing geoscientists from all over the world with high precision data from extremely small samples. "With this technology we can determine the composition of tiny areas on sub-millimetre samples with great precision," says Wiedenbeck. Thus, less than one billionth of a gram from a laboratory produced diamond needed to be removed using a very precisely targeted ion beam. Electrically charged atoms were then injected into a six metre long apparatus which separated each the billions of particles based on their individual mass. This technology makes it possible to separate chemical elements, and in particular it is possible to distinguish their lighter or heavier variants known as isotopes. "In this way we have shown that the ratio between the carbon isotopes 13C to 12C behaves exactly according the model developed by our colleagues in Novosibirsk. With this, we have contributed to the final piece of the puzzle, so to speak, to confirm this theory," says Wiedenbeck. However, it must be noted that this new method is not suitable for the mass production of large artificial diamonds.
"Our results clearly show that electric fields should be considered as an important additional factor that influences the crystallisation of diamonds. This observation may prove to be quite significant for understanding carbon isotope ratios shifts within the global carbon cycle," Yuri Polyanov sums up.
Original study: Y. N. Palyanov, Y. M. Borzdov, A. G. Sokol, Y. V. Bataleva, I. N. Kupriyanov, V. N. Reutsky, M. Wiedenbeck, N. V. Sobolev, Diamond formation in an electric field under deep Earth conditions. Sci. Adv. 7, eabb4644 (2021). DOI: 10.1126/sciadv.abb4644
Scientific contact:
Dr. Michael Wiedenbeck
Head of SIMS Facility, Section Inorganic and Isotope Geochemistry
Helmholtz Centre Potsdam
GFZ German Research Centre for Geosciences
Telegrafenberg
14473 Potsdam
Phone: +49 331 288-1484
Email: michel.wiedenbeck@gfz-potsdam.de
Media contact:
Dr. Uta Deffke
Public and Media Relations
Helmholtz Centre Potsdam
GFZ German Research Centre for Geosciences
Telegrafenberg
14473 Potsdam
Phone: +49 331 288-1049
Email: uta.deffke@gfz-potsdam.de











![[Translate to English:] Torsten Sachs in front of a climate station on a field](/fileadmin/_processed_/3/9/csm__TorstenSachs_bearbeitet_GS_4a1365ef84.jpeg)

![[Translate to English:] left image flood at the Ahrtal: image from above, several houses are flooded; left image:: Heidi Kreibich;](/fileadmin/_processed_/4/4/csm_Bild2_9af0130e9f.png)


![[Translate to English:] Start der Vega Rakete](/fileadmin/_processed_/6/4/csm_20231201-kachel_Vega-VV23-launch_ESA-CNES-Arianespace_706716b68c.jpeg)







![[Translate to English:] Poster exhibition at the Brandenburg Hydrogen Day at the GFZ, some participants in the foreground](/fileadmin/_processed_/6/5/csm_Erster_Brandenburgischer_Wasserstofftag_GFZ_402fcec95e.jpeg)
![[Translate to English:] Group picture of the participants](/fileadmin/_processed_/9/4/csm_20231108_CAWa-Workshop-Tashkent_Gruppenbild_99ea779d8a.jpeg)
![[Translate to English:] [Translate to English:] Hörsaal](/fileadmin/_processed_/e/6/csm_H%C3%B6rsal_e21ac645fb.jpeg)


![[Translate to English:] The Delegations in the Historic Library on the Telegrafenberg. In the back there are from left to right, the Dutch Ambassador for Germany, Ronald van Roeden, the Dutch Minister for Education, Culture and Science, Robbert Dijkgraaf and the scientific director of the GFZ, Susanne Buiter.](/fileadmin/_processed_/d/b/csm_Kachel-2_9eba4b4212.jpeg)

