Struvite Crystallization
In a world of ever-growing economy and global population the recovery and immobilization of nutrients and heavy metals from municipal, agricultural and industrial wastewater streams is of paramount importance for the conservation of limited natural resources and the prevention and remediation of environmental pollution.
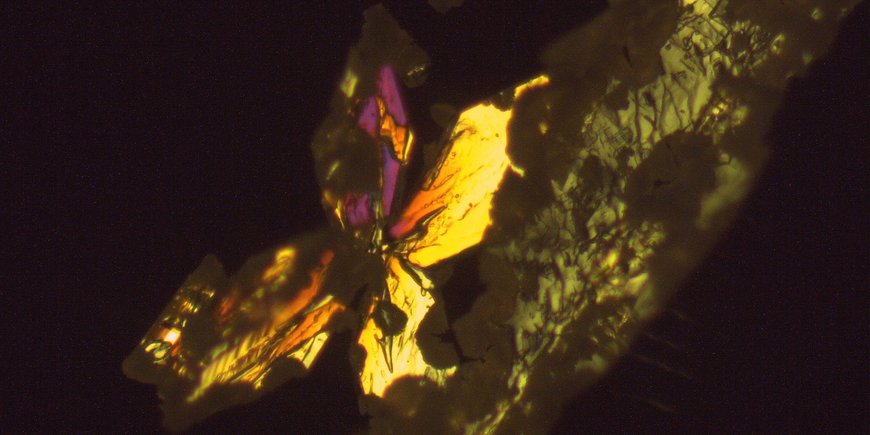
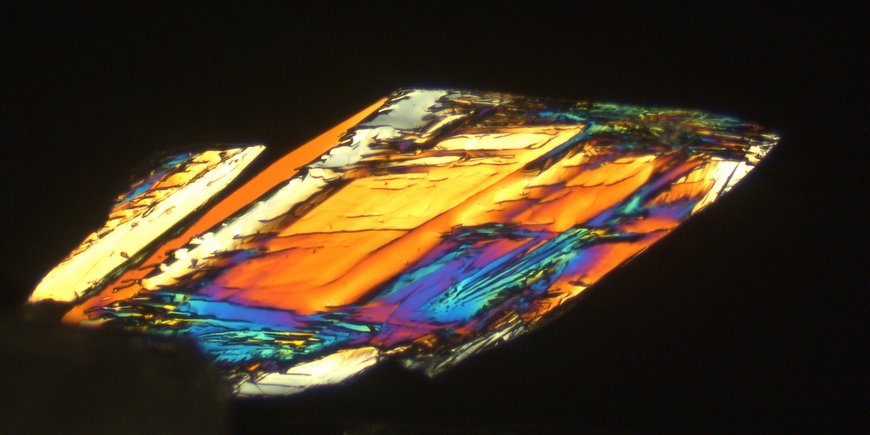
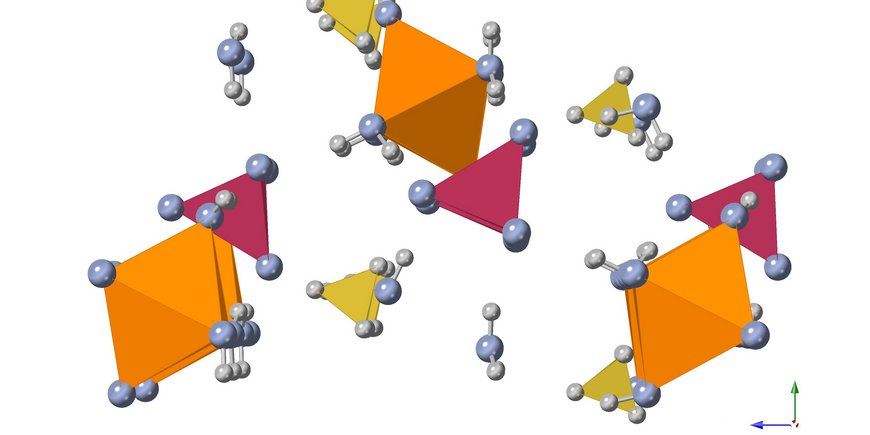
One of our current projects in this topic area explores the crystallization pathways of struvite (MgNH4PO4 · 6H2O) - one of the primary magnesium phosphates that can be extracted from wastewater - under different temperatures. A clear advantage of struvite is that it can be used as a slow-release fertilizer, combining three main plant nutrients - nitrogen (N), phosphorus (P) and magnesium (Mg) - in its crystalline structure at once. In the laboratory, we synthesize struvite crystals under various environmentally relevant conditions and follow all crystallization steps from the earliest nucleation stages to the final crystal using various in-situ and ex-situ imaging, spectroscopy and X-ray scattering methods (e.g. powder X-ray diffraction, Raman spectroscopy, SAXS/WAXS).
Another research focus is the transformation of struvite in air to the phases newberyite (MgPO3(OH) · 3H2O) and dittmarite (MgNH4PO4 · H2O), by which nitrogen and water are lost. The processes are characterized by optical microscopy, Raman spectroscopy and ex-situ X-ray diffraction of powder samples and single crystals. The research results are expected to provide a fundamental understanding of struvite crystallization pathways and conversion mechanisms, which may ultimately aid in the development of more efficient P and N recovery systems and provide implications for the use of struvite as fertilizer.
Recent Publications
(authors from the group in bold)
Hövelmann, J., Stawski, T., Freeman, H., Besselink, R., Mayanna, S., Perez, J.P.H., Hondow, N.S., Benning, L.G. (2019). Struvite crystallisation and the effect of Co2+ ions. Minerals, 9, 9, 503. DOI: 10.3390/min9090503
Hövelmann, J., Stawski, T., Besselink, R., Freeman, H., Dietmann, K. M., Mayanna, S., Pauw, B. R., Benning, L. G. (2019). A template-free and low temperature method for the synthesis of mesoporous magnesium phosphate with uniform pore structure and high surface area. Nanoscale, 11, 14, 6939-6951. DOI: 10.1039/C8NR09205B