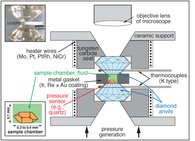
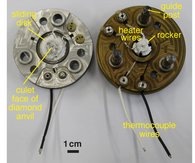
The hydrothermal diamond-anvil cell (HDAC) can be applied to study fluids and solids and particularly aqueous samples in situ at high pressures and temperatures. Although such cells are usually used at P-T conditions of less than 3 GPa and 850 °C, experiments with aqueous fluids to 23 GPa and 1025 °C have been reported in the literature. Because diamonds are transparent over a large range of the electromagnetic spectrum, samples in the HDAC can in principle be studied using any “photon-in – photon-out” method including optical techniques (microscopy/microthermometry, and infrared absorption, Raman, Brillouin, or luminescence spectroscopy) or X-ray techniques such as diffraction, fluorescence, absorption and inelastic scattering. These cells permit to obtain information on physical and chemical properties of materials that are altered upon quench, e.g., density, viscosity, sound velocity, electrical conductivity, phase transitions, complexation and speciation, solubility and partitioning, or the kinetics of mineral-fluid and fluid-fluid interaction.
The currently used hydrothermal diamond-anvil cells are modifications of the original design by Bassett et al. (1993), which has the advantage of a small temperature gradient in the sample chamber. Accuracy and reproducibility of the temperature measurements are ± 0.1 to 0.2 °C for temperatures between -25 to 25 °C and better than ± 1 °C at temperatures between 100 and 600 °C. Pressure is determined from microthermometric measurements of phase equilibrium temperatures or from the shift in the wavenumber or wavelength of Raman or fluorescence lines of an optical pressure sensor.


